
Author: Doug Dollemore
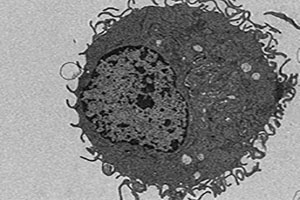
A mouse macrophage transforms heme, a potentially toxic compound, into harmless crystals called hemozoin (black spots). It's the first time that this process has been shown to occur in mammals.
Scientists are seldom effusive about their research. “Amazing” and “fantastic” just aren’t part of their vocabulary. But every once in a while, a finding is so compelling or surprising that they throw caution to the wind.
So, when University of Utah Health hematologist John D. Phillips, Ph.D., said he recently worked on a study that was “pretty cool,” it piqued interest.
The study, believed to be the first-of-its-kind, revealed that mice can detoxify excessive amounts of heme, an iron-containing component of red blood cells, using a process previously only found in blood-feeding parasites. This discovery could eventually lead to new treatments for anemia, hemochromatosis, and other red blood cell diseases.
The international team of researchers, including Phillips, a professor of internal medicine at U of U Health, set out to study how heme is recycled in the body.
“This is a fundamental process. If it happens in a mouse, it will happen in a human.”
Heme is essential for life, binding to and transporting oxygen throughout the body. Each red blood cell contains billions of these molecules. When red blood cells reach the end of their lifespan, they are destroyed by scavenger cells called macrophages. However, the heme and iron within them are reused to form new red blood cells.
Yet, paradoxically, heme is also highly toxic and can cause severe tissue damage and even death if it isn’t recycled properly. Normally, macrophages do this job really well, reprocessing about 5 million damaged red blood cells per second. But how they do it was something of a mystery.
Previous research suggested that macrophages were able to degrade hemoglobin. However, the new finding shows that this process uses a protein called HRG-1 to move heme into a compartment within the cell that can degrade it. To confirm this, Rini H. Pek, Ph.D., of the University of Maryland in collaboration with Phillips and others, disabled or “knocked out” the gene responsible for HRG-1 in laboratory mice.
If HRG-1 is a vital part of the blood iron recycling process, removing the gene should have caused a build-up of toxic heme and ultimately death.
Instead, something remarkable happened. Rather than dying, the mice survived. In fact, these mice continued making healthy red blood cells as long as they received a diet rich in iron. And they did it even though toxic levels of heme were building up in their spleens, bone marrow and livers.
Intrigued, Phillips and his colleagues dug deeper and found that the HRG-1 deficient mice were using a process similar to that used by blood-borne parasites to neutralize the potentially lethal effects of heme.
Parasites, such as the one that causes malaria, use hemoglobin within red blood cells to help replicate. But they have no mechanism to degrade the released heme and recycle the iron into the bloodstream. This excess free heme is not only harmful to the host but also the parasite itself. As a protective mechanism, the parasite has a way of coping with this problem. It transforms the hazardous heme into benign crystals called hemozoin.
Surprisingly, Phillips and his colleagues discovered that the mice in their experiments survived because they produced these crystals, too. It’s the first evidence that mammals can form hemozoin crystals and block the toxic effects of free heme. Next, researchers will need to investigate whether humans can produce these crystals if they have faulty HRG-1 genes. The team is confident this will be proven and are working to identify a study population to confirm this hypothesis.
“This is a fundamental process. If it happens in a mouse, it will happen in a human,” Phillips says.
If proven true, the researchers postulate that it could eventually lead to new treatments for certain blood disorders.