Author: Amnon Schlegel
Scientists identified a gene that controls how the liver communicates with the pancreas, which offers a new approach to combat diabetes.
Twenty-seven million Americans have type 2 diabetes, while an additional 84 million are pre-diabetic, a condition of elevated blood sugar. Both conditions are associated with cascading health problems, including heart disease, high blood pressure, and eye and kidney ailments. Neither condition can be tied solely to diet and lifestyle. Researchers at the University of Utah Health are looking for genetic factors that could predispose a person to diabetes. Their results were published in a June issue of Cell Reports.
The FOXN3 gene is one of dozens of genes associated with higher blood sugar that was identified during large population-based genetic studies. Amnon Schlegel along with a multi-institution team of scientists examined a variation in the FOXN3 gene to understand how it regulates glucagon, a hormone formed in the pancreas that promotes the breakdown of glycogen to sugar (glucose) in the liver.
“Fifty years ago, people realized glucagon was a major driver of high blood sugar, but then research attention turned to other pathways and tissues,” begins Schlegel, associate professor in Internal Medicine at U of U Health and senior author on the study. “In this project, we identified a new factor in the liver that can be influenced by and in turn influence glucagon production.”
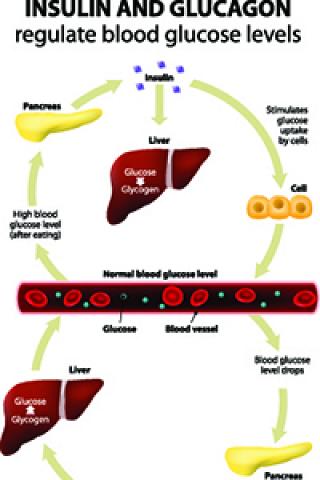
In essence, the pancreas and the liver talk back and forth. The liver makes sugar in response to glucagon but it also directs glucagon biology, which takes place in the pancreas.
In a step-by-step exploration, the researchers used animal models and human physiology studies to investigate the role of the FOXN3 gene in the development in diabetes.
They created a line of zebrafish that suppressed the FOXN3 gene. When fed a normal diet, these fish had lower blood sugar and glucagon levels compared to wildtype fish.
According to Santhosh Karanth, hypothesis driven animal studies may point to mechanisms that lead to the diseased state observed in the people carrying a genetic mutation.
“In a 2016 study, we observed that zebrafish overexpressing FOXN3 in the liver from birth have higher blood glucose,” said Karanth, PhD, research associate in the Molecular Medicine Program at U of U Health and first author on the paper. “In 2018, we observed that zebrafish lacking a copy of FOXN3 from birth have lower blood glucose.”
The team also examined the role of this gene variation in 400 non-diabetic adult volunteers. The participants underwent a 2-hour, 7-sample oral glucose tolerance test to measure blood sugar (glucose), insulin, C-peptide, and glucagon levels. While those with one copy of the SNP variant associated with higher blood glucose in the larger, initial study showed slightly higher blood sugar levels, the other parameters remained unaffected. Participants with two copies of the SNP variation showed further increase in fasting blood glucose and the also showed a diminished suppression of glucagon by the orally administered glucose.
“This gene in normal people is a determinant of blood sugar in the fasting state,” Schlegel said.
“Now that we know what FOXN3 does, we can look for targets to develop new therapies to block glucagon action to control diabetes.”
While these results are encouraging, Schlegel cautions that FOXN3 is still only one of hundreds of genes affecting healthy blood sugar.
“We don’t know for certain if this gene is predisposing people for diabetes or if it simply is setting the baseline for higher blood sugar,” Schlegel said. “It would require a larger study that includes healthy people and diabetic people to explore the role of this gene further.”
Schlegel and Karanth were joined by J.D. Adams and Adrian Vella at the Mayo Clinic College of Medicine, Lale Ozcan at Columbia University Medical Center, and Maria de los Angeles Serrano, Ezekiel Quittner-Strom, Judith Simcox, Claudio J. Villanueva, William L. Holland, and H. Joseph Yost at U of U Health.
This work was supported by the National Institutes of Health, the Juvenile Diabetes Research Foundation, and the Driving Out Diabetes Larry H. Miller Family Wellness Initiative.